| Re: Stereoisomers conformer generation and Inchikeys [message #1431 is a reply to message #1403] |
Sun, 31 October 2021 20:03   |
 nbehrnd
nbehrnd
Messages: 211
Registered: June 2019
|
Senior Member |
|
|
It should be noted that tautomers share standard an InChI and standard InChIKey in common.
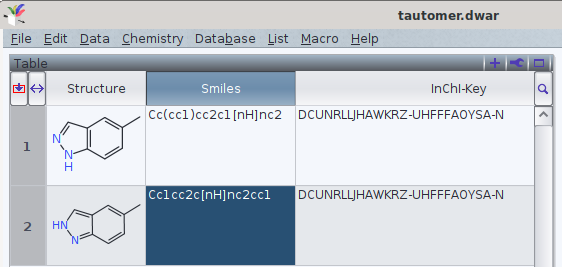
One FAQ by the InChI trust (though by 2012-05-12) explains this by
«different tautomers have the same connectivity/hydrogen layer»[1]
With the current version of InChI executables (1.06, December 2020), it is possible e.g., to add what InChI defines as «Fixed H layer», which offers to discern the tautomers. However, currently this is may yield a non-standard InChI (i.e., the string does not start with InChI=1S/, but InChI=1/). Thus, their use in a search in databases[2] may refer to more than one chemical entity.
Norwid
[1] https://www.inchi-trust.org/technical-faq-2/
[2] «Many InChIs and quite some feat» by Warr, W.A., J. Comput. Aided Mol. Des., 2015, 29: 681. https://doi.org/10.1007/s10822-015-9854-3
-
 Attachment: tautomer.dwar
Attachment: tautomer.dwar
(Size: 3.10KB, Downloaded 165 times)
-
 Attachment: tautomer.png
Attachment: tautomer.png
(Size: 23.80KB, Downloaded 598 times)
|
|
|
|
