| Re: Doubly Substituted Reactants for Combinatorial Library [message #1322 is a reply to message #1321] |
Thu, 24 June 2021 06:46   |
 nbehrnd
nbehrnd
Messages: 211
Registered: June 2019
|
Senior Member |
|
|
Dear sn,
from the documentation and some limited testing with DW I infer that this (currently) is not yet possible this way. Perhaps a misunderstanding by mine, but I infer «any atom number» is about «at this position, the atom may be a carbon, a nitrogen, an oxygen, etc», rather than the number of atoms (like let there be one atom, or two, three, etc.). On the other hand, one may prepare a list of this e.g., diamines separated by one, two, three methylene groups in advance. Past mapping the reaction in the tab «generic reaction», the subsequent tab «reactants» then allows to add them from a e.g., from a .dwar, .sdf, .mol2 file.
As an example, a bis-substitution like defined by
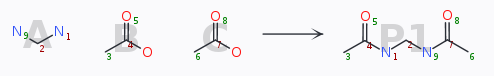
yields a list like
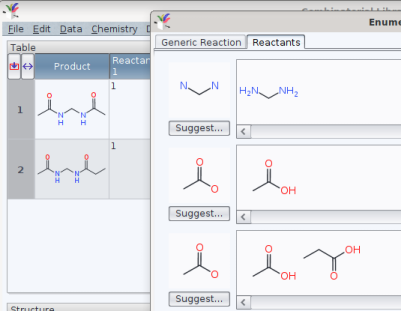
To obtain the special permutation «use the same acid on the left and on the right hand amine» would then justify to load and re-use such a pre-defined list to cover the chemical space efficiently.
Norwid
-
 Attachment: setup.png
Attachment: setup.png
(Size: 4.74KB, Downloaded 409 times)
-
 Attachment: example.png
Attachment: example.png
(Size: 28.09KB, Downloaded 436 times)
|
|
|
|
