| Stereoisomers conformer generation and Inchikeys [message #1388] |
Tue, 31 August 2021 17:08  |
 mattiafelice.palermo
mattiafelice.palermo
Messages: 3
Registered: June 2021
|
Junior Member |
|
|
Dear Thomas,
I'm not 100% sure this is a bug as I am not particularly educated about InChIKeys generation and I am a bit rusty regarding stereochemistry.
I have calculated the lowest energy conformers for a molecule with two cis/trans stereocenters (limiting to 1 conformer per stereoisomer):
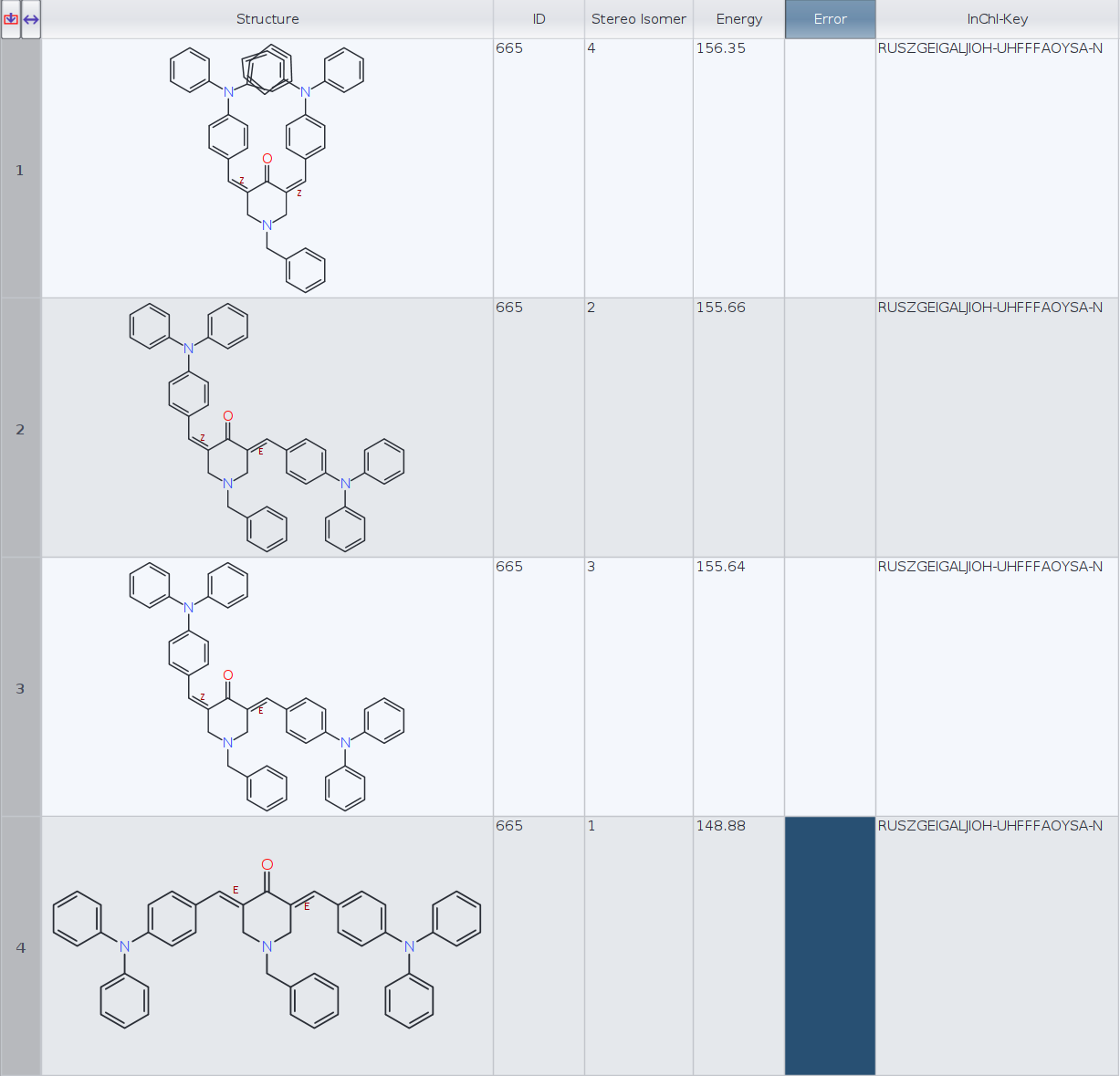
Datawarrior automatically generates the 3D geometry for four stereoisomers. Then I have generated the InChIKeys for the four molecules and Datawarrior returns the same key, RUSZGEIGALJIOH-UHFFFAOYSA-N, for every molecule (see figure).
I have tried generating the InChIKeys with RDKit in Python and instead, I have obtained the following:
ID | InChI-Key | Energy
1) RUSZGEIGALJIOH-MRSMTKAOSA-N 156.35
2) RUSZGEIGALJIOH-JYYJLMHASA-N 155.66
3) RUSZGEIGALJIOH-JYYJLMHASA-N 155.64
4) RUSZGEIGALJIOH-LMKHWIEJSA-N 148.88
I have two questions:
- Which InChIKeys are the correct ones, DataWarrior or RDKit?
- Aren't molecules 2 and 3 (E, Z and Z, E) the same stereoisomer in this particular case? RDKit InChIKeys seem to confirm that. If that is the case, perhaps DW conformer routine should only keep one of the two, or is this an intended behaviour?
Thank you for your help!
Mattia
|
|
|
|
