| Re: Generate 2D atom coordinates gives overlapping atoms [message #1849 is a reply to message #1848] |
Fri, 10 February 2023 23:00  |
 nbehrnd
nbehrnd
Messages: 224
Registered: June 2019
|
Senior Member |
|
|
Dear mcmc,
I do not recall if one can toggle on/off the stereochemical descriptors in DW's sketchers. If absent temporarily, the representation of the example from COD would be even lighter / less stuffed, than now (compared to the result by MarvinJS).
On the other hand, departing from a 3D structure imported by read of a .sdf, picking the best perspective to flatten the representation into 2D possibly stays a difficult task (option a, the example with MarvinJS). On the other hand, I speculate shredding the 3D information about coordinates to obtain a reduced representation as a string (e.g., SMILES, or DW's idnode) as an intermediate which subsequently is used to sketch the molecular structure from scratch may yield less overlaps (option b). Based on the comparison of the results, possibly DW takes this second route b.
For the COD compound, DW assigns CN(C)S(NC(c(cc1)cc2c1c(C1CCCCC1)c(-c(cc1)c([C@@H]3C4)cc1OC)[ n]2C[C@]34C(N1[C@@H]2C[NH](C)C[C@H]1CC2)=O)=O)(=O)=O.[O].[Cl ] as SMILES string. Depending if CIP labels are enabled, or not; and groups are abbreviated, or not (e.g. cyclohexyl as Cy), the implemented algorithm by CDK Depict yields
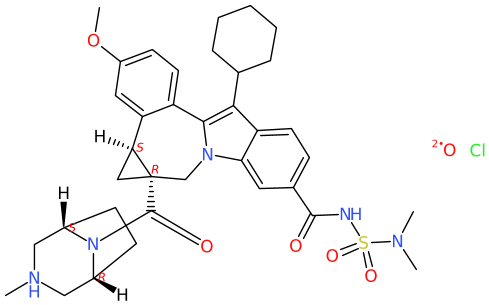
Obviously, the structure formula includes an overlap, though the absence of the wedges is indicative enough to recognize that this. Bond lengths are not uniform. The project's choice for «smart chiral Hydrogens» arguably keeps the inner of the cyclopropane moiety and bridged piperazine less crowed.
With regards,
Norwid
Web site: https://www.simolecule.com/cdkdepict/depict.html
source code: https://github.com/cdk/depict
|
|
|
|
